Introduction
Myeloid malignancies are a group of clonal hematopoietic disorders including acute myeloid leukemia (AML), myeloproliferative neoplasms (MPN), myelodysplastic syndromes (MDS), chronic myeloid leukemia (CML), chronic myelomonocytic leukemia (CMML), and juvenile myelomonocytic leukemia (JMML). These diseases are heterogenous and genetically complex and the number of genetic alterations associated with them continues to grow. To address the need for comprehensive genetics research we developed the Oncomine TM Myeloid Assay and the Oncomine TM Myeloid Assay GX v2, which both detect genomic alterations associated with myeloid malignancies including 45 DNA genes and >30 fusion driver genes with the ability to detect >700 fusion isoforms. Our panel includes genetic alterations in AML, MPN, and MDS such as FLT3-Internal Tandem Duplications (ITDs), IDH1/2, CEBPA, CALR, and TP53.
Methods
Here we describe the genomic profiles of 8503 clinical research samples (including AML, MPN, MDS, CML, CMML, or JMML). A total of 4723 samples were run on the Ion GeneStudio TM S5 System and analyzed using the Oncomine TM Myeloid Research workflow on Ion Reporter TM 5.18. A total of 3780 samples were run on the Ion Torrent TM Genexus Software 6.6 and analyzed using the Oncomine TM Myeloid Assay GX v2.
Results
The turnaround time (the time between starting the run and NGS data report) was 23-25 hours and the hands-on time was around 1 hour, which enabled fast and accurate profiling of myeloid malignancies, and facilitated the timely availability of results. The success rate of the samples was 100%.
Frequency of relevant mutations by gene
Some genes had a higher rate of genetic mutations. TET2 has the highest percentage of detection (12.6%, 1068 positive sample/8503 total number of samples). Other important genes with the highest percentage of detection included ASXL1 9.3% (787/8503), DNMT3A 7.8% (665/8503), TP53 7.5%, (640/8503), SRSF2 6.9% (587/8503), JAK2 6.6%, (562/8503), RUNX1 5.2% (446/8503), SF3B1 5.0% (423/8503), IDH2 4.0% (337/8503), NRAS 3.8% (318/8503), FLT3 3.4% (292/8503), ABL1 3.2% (273/8503), BCOR 2.7% (229/8503), NPM1 2.4% (201/8503), and IDH1 1.0% (170/8503). Overall, 4797 (56.4%) of samples had a mutation in at least one gene in the panels, and the average number of mutations per samples was 2.3 within these samples. The most co-occurring gene pairs include ASXL1 and TET2, as well as SRSF2 and TET2, both are detected in more than 2% co-occurring gene pairs.
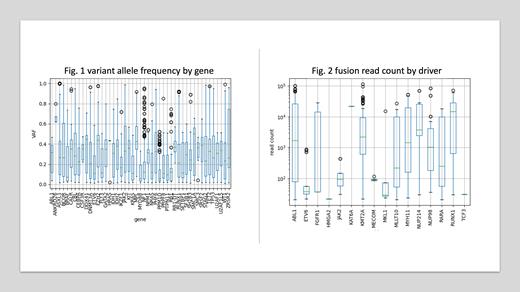
Variant allele frequency by gene
We observed a substantial difference between the variant allele frequency (VAF) of relevant DNA mutations (SNP + Insertions + Deletions) in different genes. ANKRD26 has a median VAF of 66%, whereas MYD88 has a median VAF of only 7%. (Fig.1)
Mutation spectrum of FLT3ITD variants
We observed FLT3-ITD in 3% of samples. We observed the VAF of FLT3-ITD variants averaged around 0.32. Interestingly, the length of the FLT3-ITD duplication is multi-modal, with the highest peak at around 34 bp. The maximum duplication length detected is 151 bp.
Mutation spectrums of fusions
Of the 8480 samples with RNA fusion profiling, 710 were fusion positive (8.4%). ABL1 is the most common fusion driver (432 samples, 5.1%), with 88% of these being BCR- ABL1. Other common fusion drivers include KMT2A (171 samples, 2.0%; 29% are KMT2A-MLLT3 and 26% are KMT2A-MLLT1), MYH11 (126 samples, 1.5%; all are CBFB-MYH11), RARA (117 samples, 1.4%; all are PML-RARA), RUNX1 (95 samples, 1.1%; 56% are RUNX1-RUNX1T1). A total of 17 distinct driver genes, 49 distinct gene pairs and 110 distinct gene isoforms were observed, including rare fusions (for example, ZMYM2-FGFR1, KAT6A-CREBBP, etc) that are not detectable by traditional methods like qPCR (quantitative polymerase chain reaction) or FISH (fluorescence in-situ hybridization). The read count of the fusion positive samples ranges from 21 to 113,000. (Fig.2)
Conclusions
The Oncomine Myeloid Assay is a fast, accurate, robust, and reproducible solution for comprehensive genomic profiling of myeloid malignancies. We describe the mutational spectrum of DNA variants and RNA fusions in a range of clinical research samples, exploring the VAF, the abundance of mutations in different genes, and the properties of observed gene fusions.
(For research use only. Not for use in diagnostic procedures.)
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal